ISO 15189:2012
What is ISO/IEC 15189 Clinical Test Lab Accreditation?
ISO 15189 Medical Laboratories — Particular requirements for quality and competence is a ‘variant’ standard derived from ISO/IEC 17025 with specific focus on clinical laboratories. The standard covers an ‘end-to-end’ approach to the application of quality assurance methodologies in a clinical environment. This range from definition of services offered through to collection of patient samples, sample transport, storage, transportation and test and includes the reporting, interpretation and delivery of test results. The standard also focuses on management responsibilities as well as supporting processes such as facilities, calibration, method validation, training etc.
ISO 15189:2012 IS THE INTERNATIONAL STANDARD FOR MEDICAL LABORATORIES – REQUIREMENTS FOR QUALITY AND COMPETENCE.
Within the healthcare sector as in most other areas of industry and commerce, the need for organisations to operate formal Quality Management Systems is fast becoming a Commissioner, Service User, and/or Regulatory Authority prerequisite.
Some of the other common healthcare standards currently in place include external certifications such as ISO 22870, ISO 17043, ISO 13485, MHRA, HTA, Imaging Services Accreditation Scheme-ISAS many others.
ISO 15189:2012 can be used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies. ISO 15189 is very robust and heavy with precise requirements.
ISO 15189 quality standards provide an effective quality management and technical competence framework that enables medical laboratories to meet international standards for patient care and laboratory responsibility. The ISO 15189 quality standards, when carefully planned to meet laboratory requirements, can also improve laboratory services, products and business processes.
Medical laboratories can also benefit from the automation of business processes and controls that help obtain or continually maintain ISO 15189 quality standards.
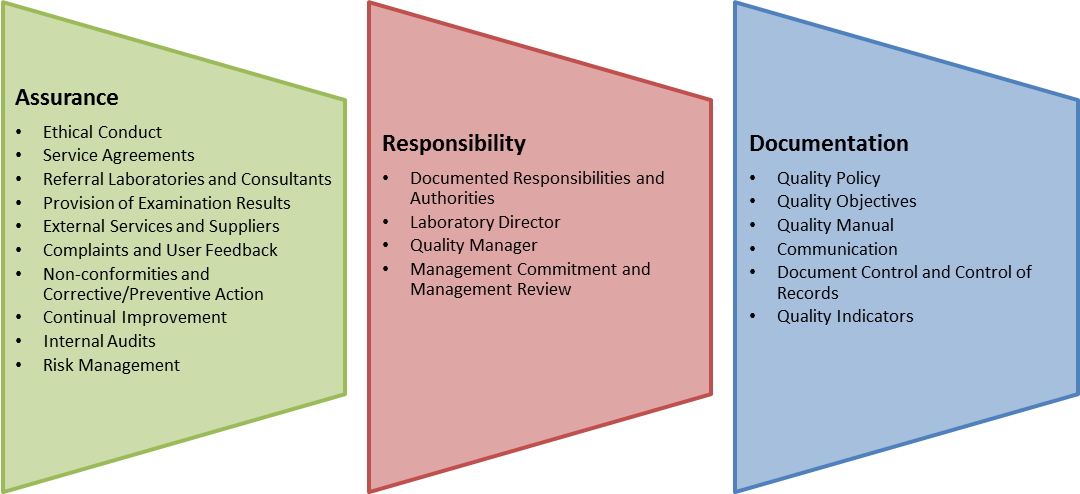
The following are the basic requirements for ISO 15189 certification:
- A well-documented procedure analysis by the laboratories.
- Training manual provided by the laboratories.
- ISO 15189 requires an effective detailed analysis of medical laboratory procedures in a bid to make sure that all weaknesses have been identified.
- Detailed evaluation reports of the existing quality management system as well as other monitoring and evaluation reports.
- A detail audit of management reviews The ISO 15189 certification has a set of very unique benefits and the main objective of certification is quality assurance.
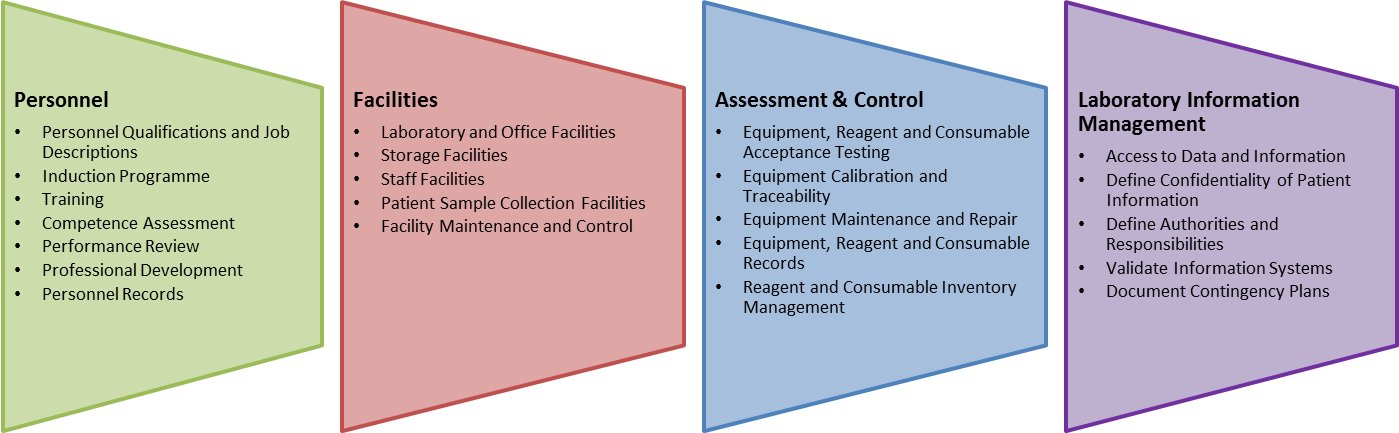
The benefits are listed here below:
- An ISO 15189 certification promotes development of an international reputable image for your organization through Quality Assurance and Management Systems.
- Promotes a strong degree of consistency in compliance to medical laboratory laws and legislation both from an international and national perspective.
- Fundamentally critical in promoting laboratory efficiency and responsibility for better results and service delivery.
- The certification program also has been very critical in promoting modern laboratory practices that include professionalism and expertise in conduction of medical activities in the labs.
- Ultimately promoted the growth of customer satisfaction in organizations involved in medical laboratory testing.
Some key benefits include:
- Recognition of testing competence – as mentioned above, customers can recognise the competence of a lab with an internationally recognised standard.
- Marketing advantage – accreditation can be an effective marketing tool as labs can demonstrate their quality and overall competence.
- Benchmark for performance – laboratories can determine whether they are performing to the appropriate standards and provide them with a benchmark to maintain that standard.
Get in Touch! Ask us any question/query on +91-9867-180-395. We would be happy to answer your concerns. You can also drop an email at info@ascentinspecta.com
